What Best Describes the Electron Cloud Model
The negative charge of the electron. Erwin Schrodinger an Austrian physicist came up with the electron cloud model in 1926.

What Is The Electron Cloud Model Universe Today
The electron cloud model describes which of the following.

. These energy levels can only hold a certain number of electrons at any time and the organizations of these electrons in the electron cloud 2e. This assessment will help you. Which of the following best describes the electron cloud model.
It shows that electrons usually carry a negative charge. C It is a region in space in. Answered expert verified.
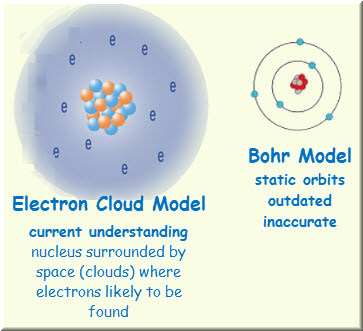
The Electron Cloud Model. Most current model of the atom Electron cloud model is used to describe where electrons are when they go around the nucleus of an atom To its credit the Bohr Model shows where electrons have the highest probability of being at any given moment so while the electron cloud model is the most accurate way to depict an atom with simplicity it doesnt make the other options. The Electron Cloud Model was of the greatest contributions of the 20th century leading to a revolution in physics and quantum theory.
The shape not the size of an electron cloud is determined by the electrons ____. The electron cloud refers to a region outside the nucleus where an electron is most likely to be found. She has taught science courses at the high school college and graduate levels.
The group of protons that are in the nucleus. Which statement best describes the electrons in this illustration. The electron cloud model says that we cannot know exactly where an electron is at any given time but the electrons are more likely to be in specific areas.
B It is a region in space that has a precise shape and is completely filled hva dense electron cloud. Unit 4 Test Review DRAFT. The negative charge of the electron.
Helmenstine holds a PhD. The trails left by an electron as it moves around the nucleus. It tells us that electrons are found in defined energy states in outside of the nucleus of the atom.
The electron cloud model describes which of the following. The electron cloud is the location around the nucleus that contains negatively-charged electrons. Which statement about the electron-cloud model is true.
The electron cloud shows the area in space where an electron is most likely to be. How strong a magnetic field must be experienced by the electron if its path is a circle of radius 55 cm. PLUM PUDDING MODEL OF THE ATOM D.
Electron cloud model is a model of an atom in which the atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electronsThe electron cloud model says that we cannot know exactly where an electron is at any given time but the electrons are more likely to be in specific areas. Electron cloud model is a model of an atom in which the atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Before understanding what an electron cloud model is it is important to know about the forces that bind the electrons together.
Understand the Electron Cloud Model. The electron cloud. It shows that electrons remain in high-energy subshells.
In the electron cloud model of the atom what does the cloud represent. SOLAR SYSTEM MODEL OF THE ATOM B. Which statement best describes an occupied orbital according to the quantum mechanical model.
The group of protons that are in the nucleus. The shape not the size of an electron cloud is determined by the electrons ____. The cloud is denser where you will probably find an electron.
BILLIARD BALL MODEL OF THE ATOM C. The electron cloud is used to describe the behavior of electrons and it is useful in building a model of the atom. What is the electron configuration of this atom.
The probability function basically describes a cloud-like. The potential V between two charges is best described by a Coulomb termVr 4 π ϵ r Z e 2 where Ze is the charge of the nucleus Z1 being the hydrogen case Z2 helium etc the other e is the charge of the single electron ϵ is the permittivity of vacuum no relative permittivity is needed as the space inside the atom is empty. Which statement best describes the Lewis dot structure for fluorine.
Chemistry questions and answers. The group of protons that are in the nucleus. You might also like to know about the parts of an atom before.
Location where an electron has the highest probability of being found. The probability of the electron location. An electron cloud is a visual model of the most probable locations of electrons in an atom.
Answer in units of t. 1s2 2s2 2p6 3s2 3p6 4s1. Location where an electron has highest probability of being.
It shows that the electrons within an atom do not have sharp boundaries. In biomedical sciences and is a science writer educator and consultant. Which of the following best describes the modern atomic theory.
SOLAR SYSTEM MODEL OF THE ATOM. What is the electron cloud model. Which of the following best describes the electron cloud model.
The electron cloud model describes which of the following. The electron cloud is a theoretical model that attempts to explain the erratic behavior of electrons in an atom. The electron cloud model describes the _____ of electrons in an atom.
ELECTRON CLOUD MODEL OF ATOM 2 See answers iandinglasagmailcom iandinglasagmailcom Answer. The lowest possible state for any electron is called its ground state. Berdasarkan model atom berikut yang merupakan model atom dalton adalah.
An electron cloud is a visual model of the most probable locations of electrons in an atom. The charge on an electron is 160218 1019 c and its mass is 910939 1031 kg. A It is a spherical or dumbbell-shaped route traced by the electron in its rapid movement.
When an atom of lithium loses an electron the atom becomes a. It shows that electrons move quickly in circular orbits.

Electron Cloud Model Theory Examples What Is An Electron Cloud Video Lesson Transcript Study Com
No comments for "What Best Describes the Electron Cloud Model"
Post a Comment